
Convection – Rising heated air molecules
Convection – Rising heated air molecules According to the existing definition: Convection is the transfer of heat vertically from one place to another, by bulk


The Existing Model:
Pressure is the force exerted by one substance on another per unit area. In the existing model, the Ideal Gas Law, a kinetic theory (Figure 1) was applied, where the pressure of a gas is defined as:
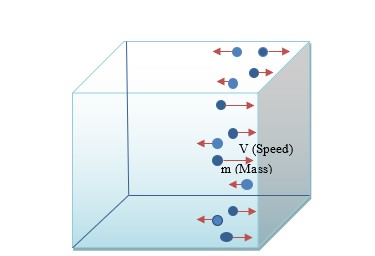
Challenges in the Existing Model:
Challenges observed in applying the kinetic theory are, the assumptions:
1. All gas molecules in a unit volume hit a unit area during a unit (period of) time.
If a fraction of the unit (period of) time is considered, pressure too has to be a fraction of the pressure of the unit (period of) time. Such does not reflect the actual situation in the nature. Pressure is a continuum property, thus has to be independent of time.
2.Perfect elastic collisions happen between gas molecules and the wall.
Perfect elastic collisions (do not lose any of their kinetic energies during collisions between objects) do not exist in reality. It is not a realistic assumption, evidently, a too hypothetical notion deployed to explain the observations.
3. All molecules hit the wall perpendicularly.
In reality, as molecules move in random directions; change of momentum of each molecule against the wall is not the same.
4. All molecules move in one direction
This would not cause pressure in the perpendicular directions. Such does not reflect the actual situation in the nature, where the pressure of a gas at a given point is the same in all directions.
The perspective that momentum change of molecules causes pressure, yields an unrealistic narration which is an attempt to explain the observation; instead of focusing on the phenomenon. It fails to adhere to accepted scientific norms in giving a closer to reality answer explaining the cause of the pressure in gases!
Force Field on Molecules:
In the Force Field perspective, all the hypothetical assumptions made under the kinetic theory excluded. Pressure is explained based on the force field. A force field is not time dependent, hence more successful in explaining a continuum property.
The pressure in gas can easily be explained as the resultant of:
among gas molecules. This perspective will provide a better explanation of the phenomenon.
Although some phenomena observed in the nature/universe, e.g., pressure and expansion, are dependent on the temperature which is a manifestation of the thermal energy content, none of the four fundamental forces in existing models are considered dependent on the thermal energy content/temperature. As presented below, proven both analytically and experimentally, the thermal energy content dependent gravitational repulsion force, would fill the critical gap between energy and fundamental forces!
As gravitational attraction is caused by the mass, the gravitational repulsion is caused by the thermal energy. Please see Figure 2.
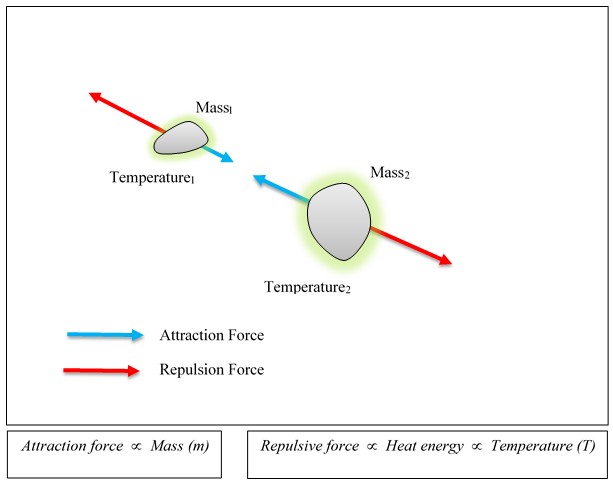
Gravitational repulsion (Antigravity) force is repulsive and proportional to the thermal energy of the matter. With the increment of the temperature, anti-gravity force is increased.
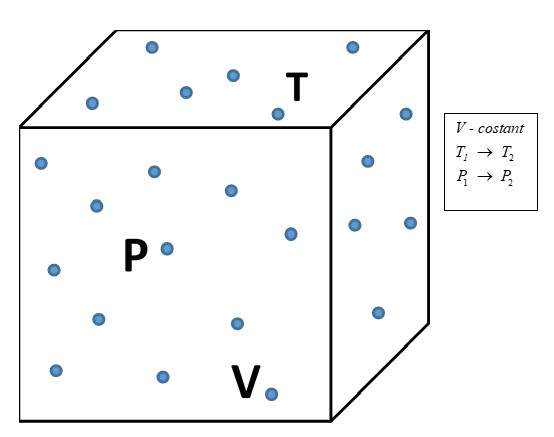
Figure 3 presents gas in a container at temperature T, volume V and pressure P.
If the distance among atoms/molecules (thereby the volume V) is kept constant, and the temperature T is changed from T1 to T2, the repulsive force, hence the pressure P, changes from P1 to P2 (Amontons’ Law for gases).
Mathematical Model for Pressure using Gravitational Repulsion and Attraction Forces:
For the mathematical analysis, consider only two molecules of mass m, containing thermal energy Q (corresponding to their temperature T). confined within a box at a distance r apart (Figure 4). A certain pressure exists; therefore, an outward force FW exists. The two molecules, hence, are at rest touching the walls of the box, under the gravitational repulsion force (FR), the gravitational attraction (FA) and the force FW exerted by the wall. Existence of pressure on the walls of the box imply that the repulsion force between molecules is greater than the attraction force (FR > FA).
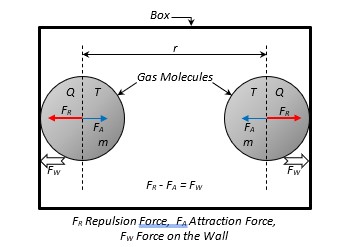
For equilibrium of forces on gas molecules depicted in Figure 4:
Fr – FA = FW ………………………………………………. (1)
In this situation, Pressure P on the wall (by the two gas molecules) results from the outward force FW of the system on a unit area; thus: P ![]() FW
FW
In reality, any small quantity of gas molecules exhibits same pressure regardless of the number of molecules enclosed. This implies that pressure at any point in the gas is caused by the very basic building block; intermolecular forces.
Established experimental data [3] (published in 1948) on thermodynamic properties of gaseous nitrogen, hydrogen, oxygen, water vapor, carbon monoxide and carbon dioxide were used in the calculations to elucidate the effects. The research yielded that, gravitational repulsion and gravitational attraction forces are colossal (larger in the order of 1032 and 1029 times respectively at around 333 K and 1 atm) than the traditionally known gravitational force between gas molecules. What has been calculated as gravity force so far according to the existing models is, the resultant of the two large forces gravitational repulsion and attraction, which are nearly equal thus nearly in equilibrium.
To read the complete report of this research, please visit the following link:
An alternative model of gravitational forces in nature using the combined effects of repulsion and attraction forces on gaseous molecules
Both gravitational attraction and repulsion (Antigravity) forces pervade our environments at both micro and macro levels. Once fully understood, such nearly in balance colossal forces offer the prospect of learning to control and manipulate to achieve hitherto unknown results, outcomes and developments. This is another direction for future research.

Convection – Rising heated air molecules According to the existing definition: Convection is the transfer of heat vertically from one place to another, by bulk

Thermal expansion & State change of matter Predominant Existing Theory A given atom may rest in its equilibrium position, r0, i.e., at the minimum of

Upward motion of condensed water droplets in still air Upward motion of water droplets when not supported by convection currents has been observed (Piyadasa, 2012),