
Accelerating expansion of universe
Accelerating expansion of universe The phenomenon ‘Accelerating Expansion of Universe’ (Perlmutter et al., 1998, Riess et al., 1998) is based on the observation that galaxies


According to the existing definition:
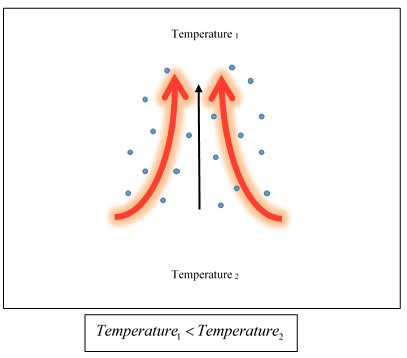
Convection is the transfer of heat vertically from one place to another, by bulk movement of molecules or particles within fluids (gases and liquids). Convection happens when the temperature of a region of a liquid or gas is greater than the region above it. Thermal expansion due to the greater temperature, causes the density of the lower region of liquid or gas to become lesser than the region above it; which causes buoyancy. Buoyancy causes upward bulk movement of fluid (Figure 1), thus transporting heat from the lower region to the upper.
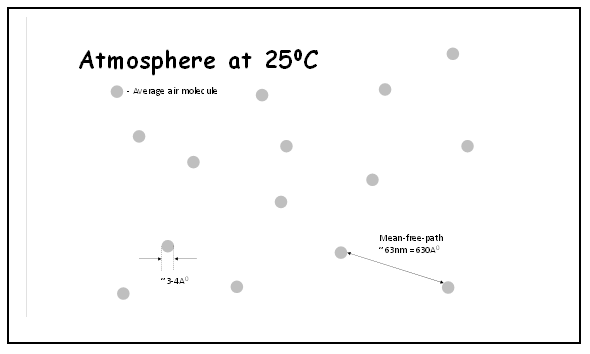
Continuing to base on existing definitions, consider air at room temperature ≈ 25°C as depicted in Figure 2. The diameter of an air molecule would be ~3-4 Å. The average distance among air molecules would be ~3.5 nm = 35 Å, if molecules are uniformly distributed in free space. Mean-Free-Path (mfp) of an air molecule at room temperature would be ~63 nm = 630 Å. These values indicate that air molecules are too far apart to interact/bond with each other; they are more likely to act as individual entities.
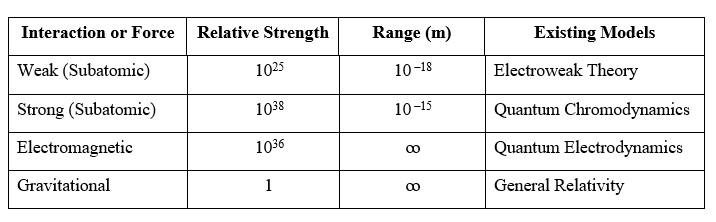
Possible forces among the molecules need to be considered as well. There are four (04) fundamental forces accepted in the existing definitions (Table 1). Therein, the gravitational force is reckoned as a “weak force”; 10–36 times weaker compared to the electromagnetic force; accordingly, a negligible force. Strong and weak forces mentioned in Table 1 act at subatomic level only.
Table 1: Fundamental Interactions (also known as Fundamental Forces in existing definitions) (Davies et al., 1986)
Analyzing the scenario microscopically, based on existing definitions, the above discussion yielded that:
Air molecules in the atmosphere, hence, cannot be considered as a bulk/primary substance. The Archimedes’ Law (the concept of buoyancy) cannot be applied, as there is no primary substance to be displaced by the air molecule. As there is no Archimedean buoyancy force applicable at molecular level in free space:
It is common experience that all air being attracted downwards to the Earth’s surface, does not happen in the atmosphere. There appears to be another force (not identified in existing knowledge) that enables air molecules to float.
Further, according to the existing definitions, change of thermal energy/temperature of individual molecules would only change kinetic energy; not change the density of individual molecules. The relationship between the fundamental forces and kinetic energy is also not available in existing definitions. (Existing definitions say that change in thermal energy/temperature would change kinetic energy of the particle, but no mechanism is given as to how it happens. Kinetic energy is believed to exist in the form of vibrational/translational/rotational/linear motions of the particles. Change of kinetic energy is believed to change Brownian motion as well; again, without a clear mechanism).
Consider a volume of air that gets heated. Therein, consider a single molecule that has received thermal energy (Figure 3). According to the existing definitions, kinetic energy (vibrational/translational/rotational/linear motions) of the molecule increases (Figure 4).
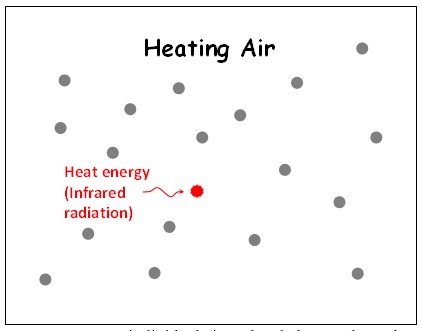
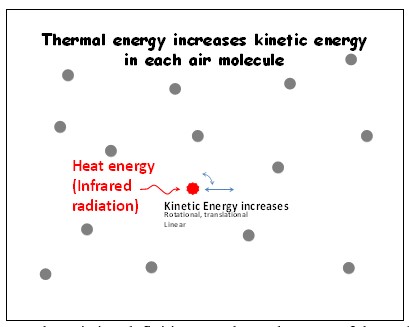
Once the thermal energy is received by the air molecule (according to the existing definitions):
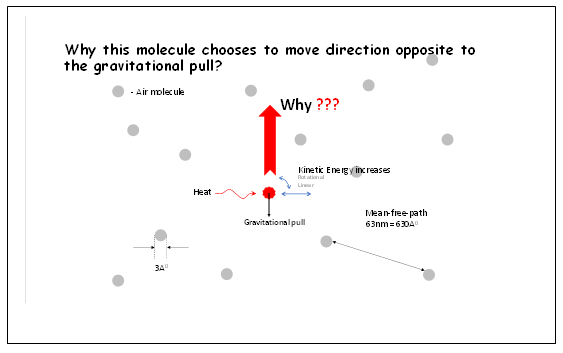
The above upward motion takes place, notwithstanding the inhibitions discussed above (according to existing definitions), viz. air molecules are:
What makes air molecules display this phenomenon of upward motion, despite all the inhibitions discussed above? Is there a temperature dependent gravitational repulsion force that is distinct and opposite to the gravitational attraction force of the Earth?
As gravitational attraction is caused by the mass, the gravitational repulsion is caused by the thermal energy. Please see Figure 6.
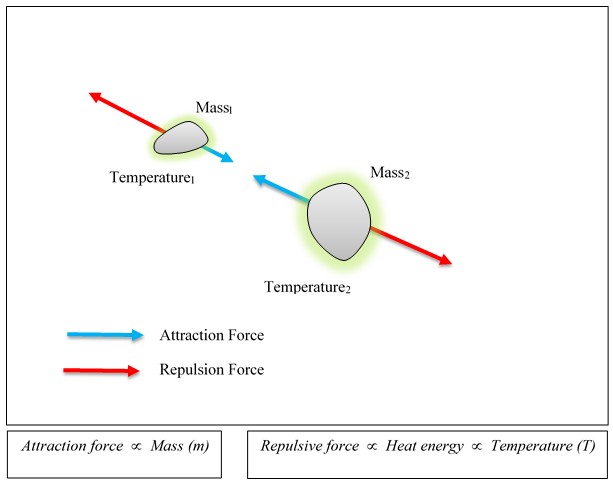
At the normal surrounding temperature, the air molecule is at dynamic equilibrium, where the gravitational attraction and repulsion forces are nearly equal. Once the temperature rises, the repulsive force with the Earth increases, and the equilibrium ceases. Equilibrium between the gravitational attraction and repulsion forces changes to a resultant force in the direction away from the Earth (Figure 5). Now the air molecule moves in the upward direction (away from the Earth).
Reference:

Accelerating expansion of universe The phenomenon ‘Accelerating Expansion of Universe’ (Perlmutter et al., 1998, Riess et al., 1998) is based on the observation that galaxies
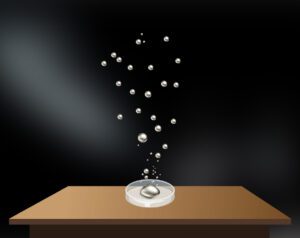
Upward motion of mercury molecular clusters at room temperature Experimentally verified following observations cannot be explained by existing theories! They could, nevertheless, be easily explained

Why do clouds float in mid-air? Do classical/conventional definitions provide answers? In meteorology, a cloud is a visible mass of liquid water droplets or frozen