

Why do clouds float in mid-air?
Why do clouds float in mid-air? Do classical/conventional definitions provide answers? In meteorology, a cloud is a visible mass of liquid water droplets or frozen


Upward motion of water droplets when not supported by convection currents has been observed (Piyadasa, 2012), (Piyadasa, 2019), as presented in Videos 1 and 2. Such phenomenon cannot be adequately explained by existing theories. Water is nearly 1,000 times denser than air. If so, why do water droplets move upwards in still air, against the gravitational pull by the earth?
Video 1. This animation shows that the path of condensed water droplet that are ejected downwards from spout of a kettle.
Video 2. Thermal image of downward projected steam. Those steam convert in to water droplets when the temperature drops at the spout (directed downwards) of the kittle.
Thermal images (Video 2 and Figure 1) present downward projected condensed steam droplets taken at room temperature (24°C) from the Cryogenically cooled third generation forward looking infrared (FLIR) thermal camera (3-5μm). As presented in Figure 1a and b (Piyadasa, 2012), there is no primary thermal energy source below the turnaround point (TAP) of water droplets. Only the radiation from the mixture of condensed steam (water) droplets and water vapor emanating from the tube, heats up the surrounding air making possible contributions towards convection. The thermal image Figure 1b shows that the temperature below TAP cannot cause conditions for convection. The lower part, between lines UV and XY shows (Figure 1c) decreasing temperature from 600C to 240C, which definitely inhibits any possible upward convection movement.
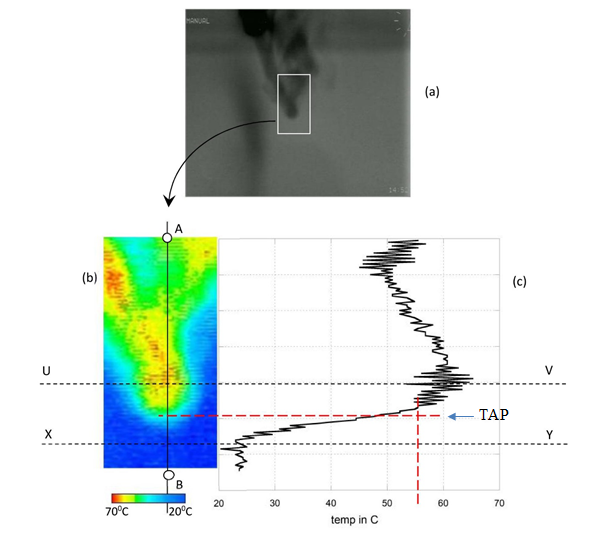
Source: “Will rising water droplets change science?” by C. K. G. Piyadasa, 2011, Canadian Journal of Pure and Applied Sciences, Vol. 6, No. 2, pp.1995, Reprinted by permission of SENRA Academic Publishers, 5919 129 B Street Surrey, British Columbia, Canada V3X 0C5.
Important points to mention related to fundamental aspects of upward motion of water droplets due to convection currents
The average dimension (diameter in this case) of a water droplet due to condensed steam at room temperature of 30°C and relative humidity around 70% is around 10µm (Piyadasa, 2012). The mean free path of air at atmospheric pressure and temperature 30°C is approximately 65 ηm (Jennings, 1988). The minimum average length d (i.e., ) of the area required to be affected by pressure is equal nearly to 42.3 µm. Water droplets that we consider in this experiment, however, are around 10 µm in diameter, which is much smaller than the minimum dimension that should prevail to be affected by the pressure due to molecular motion of air convection. Air convection, therefore, cannot affect water droplets even if convection currents do exist.
Mass of a ![]()
![]()
![]()
It is clear from the above three arguments, that:
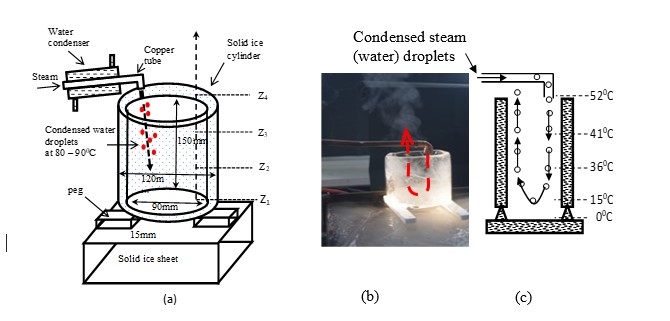
Phenomena shown Figure 1, and Videos 1 and 2 are further challenged by the experiment shown below. The temperature gradient needed for supporting/creating convection currents in air are further reduced by introducing an ice cylinder as shown in Figure 2. Notwithstanding the more challenging conditions created by the ice cylinder, condensed steam (water) droplets projected downwards turn around at some point and started moving upward (see Video 2). Please refer (Piyadasa, 2019) for detailed description.

The above discussed experimentally verified observations cannot be explained by existing theories! These observations, nevertheless, can easily be explained by considering a gravitational repulsion force in addition to the gravitational attraction force.
As gravitational attraction is caused by the mass, the gravitational repulsion is caused by the thermal energy. Please see Figure 3.
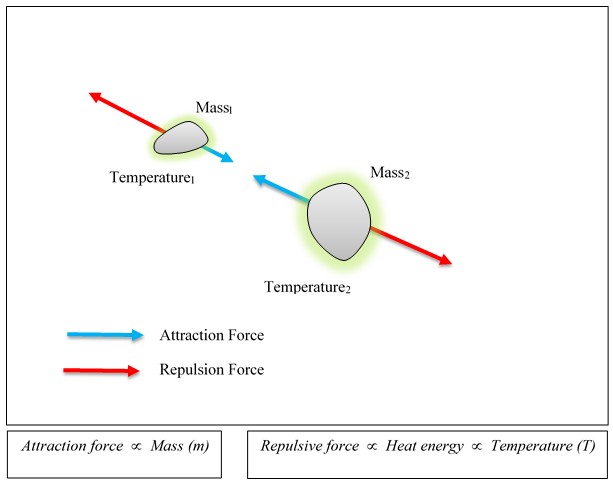

Explanation considering both gravitational repulsion (Antigravity) and attraction
Above experimental observation simply tells us that there is a cryptic force, which acts as a repulsive force (i.e. a repulsive force between the Earth and water droplets) against the direction of gravitational pull. This phenomenon can be better explained by considering both gravitational repulsion (Antigravity) and attraction.
References:


Why do clouds float in mid-air? Do classical/conventional definitions provide answers? In meteorology, a cloud is a visible mass of liquid water droplets or frozen

Upward motion of mercury molecular clusters at room temperature Experimentally verified following observations cannot be explained by existing theories! They could, nevertheless, be easily explained


What causes pressure in gases The Existing Model: Pressure is the force exerted by one substance on another per unit area. In the existing model,