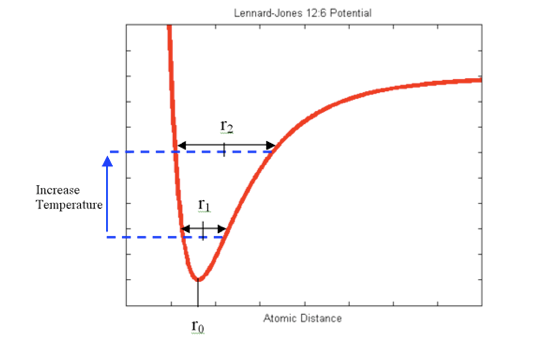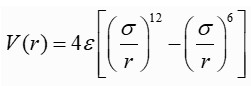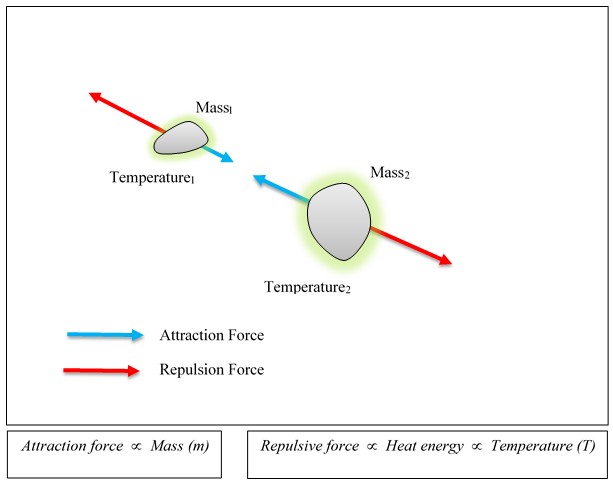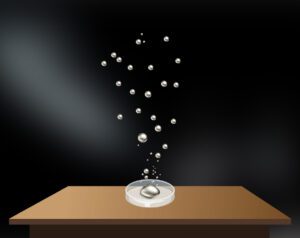
Upward motion of mercury molecular clusters at room temperature
Upward motion of mercury molecular clusters at room temperature Experimentally verified following observations cannot be explained by existing theories! They could, nevertheless, be easily explained