Points to Ponder
1.Creation and upward motion water droplets at minus winter temperatures from hot water
2. Physical state Change of Matter
When temperature is changed by changing its heat content of matter, its state changes according to the following figure at different pressures. Condensation, sublimation, vaporization, melting, deposition are some other phenomena associated with matter.
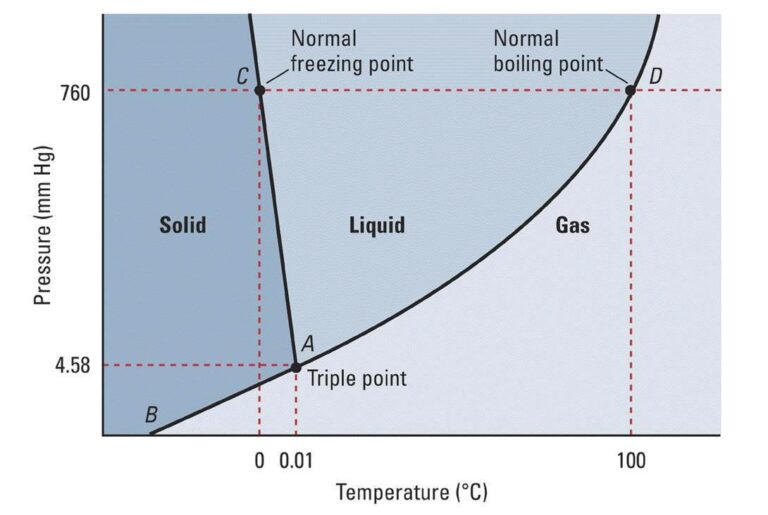
Can these processes be explained better by considering gravitational attraction and repulsive forces?
3. Surface Tensioner
Surface tension is the tendency of fluid surfaces to shrink into the minimum surface area possible.
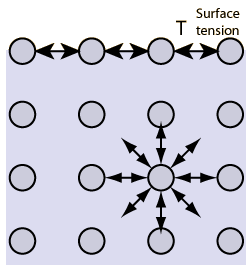
Please note that surface tension depends on temperature. See following figure as an example where surface tension depends on the temperature of water.
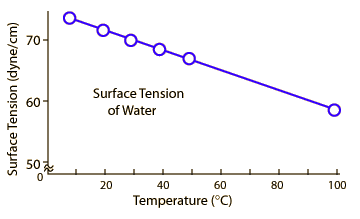
Can these processes be explained better by considering gravitational attraction and repulsive forces?
4. Nano suspension (colloid)
Nanosuspensions are nanosized submicron particles which are insoluble, suspended in dispersion (collide) in any fluid. Examples: fog, mist, smoke, pigmented ink etc,
Gold nano particles in a solution (Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy, Journal of Advanced Research Volume 1, Issue 1, January 2010, Pages 13-28)

Can these processes be explained better by considering gravitational attraction and repulsive forces?
5. Capillary Action
Existing theory suggested that Capillary action occurs because water is sticky, thanks to the forces of cohesion (water molecules like to stay close together) and adhesion (water molecules are attracted and stick to other substances). Adhesion of water to the walls of a vessel will cause an upward force on the liquid at the edges and result in a meniscus which turns upward. The surface tension acts to than the cohesive forces between the liquid molecules. The height to which capillary action will take water in a uniform circular tube is limited by surface tension and, of course, gravity, hold the surface intact. Capillary action occurs when the adhesion to the walls is stronger
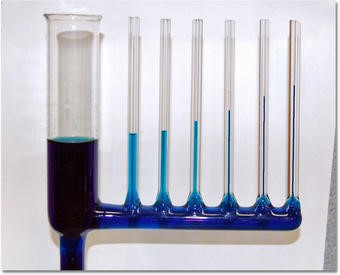
Can these processes be explained better by considering gravitational attraction and repulsive forces?
6. Heavy molecules such as CFC at higher altitudes
CFCs reach the stratosphere because the Earth’s atmosphere is always in motion and mixes the chemicals added into it. Gases such as CFCs that do not dissolve in water and that are relatively unreactive in the lower atmosphere are mixed relatively quickly and therefore reach the stratosphere regardless of their weight.
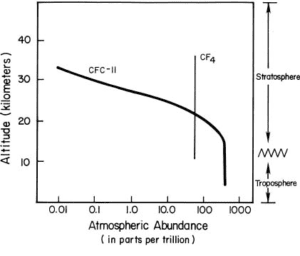
Can these processes be explained better by considering gravitational attraction and repulsive forces?
7. Viscosity
Viscosity is often referred to as the thickness of a fluid. The viscosity of a fluid is a measure of its resistance to deformation at a given rate.At a molecular level, viscosity is a result the interaction between the different molecules in a fluid. This can be also understood as friction between the molecules in the fluid. Just like in the case of friction between moving solids, viscosity will determine the energy required to make a fluid flow. stratosphere regardless of their weight. Read more ->
Can these processes be explained better by considering gravitational attraction and repulsive forces?

